Willkommen bei LimnoMar
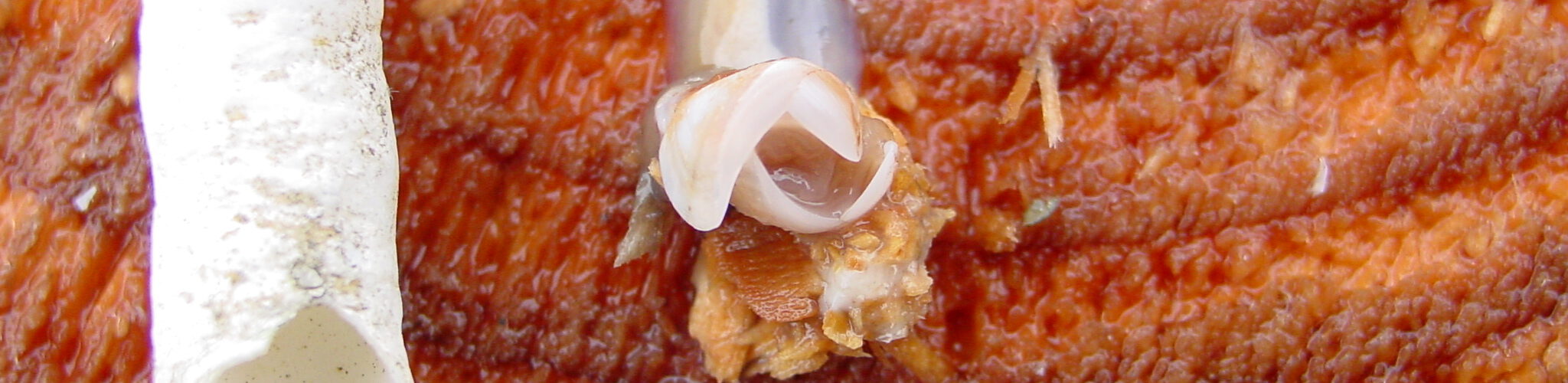
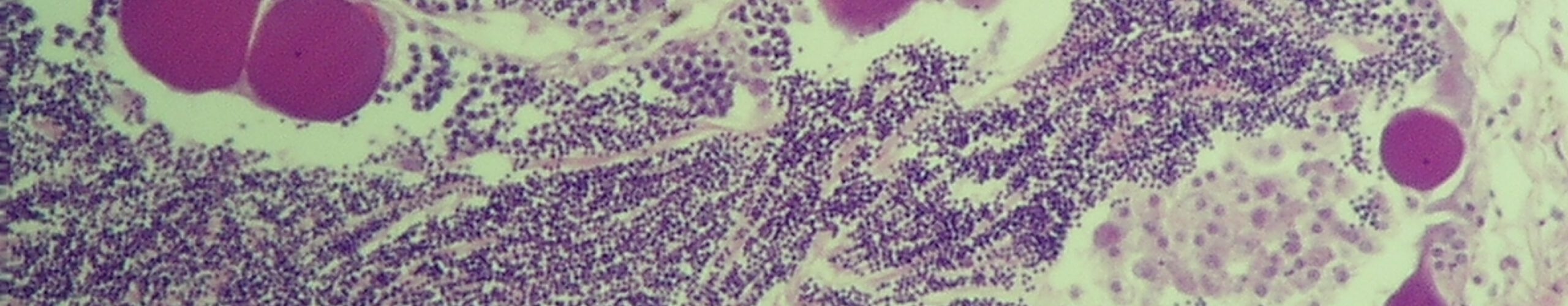
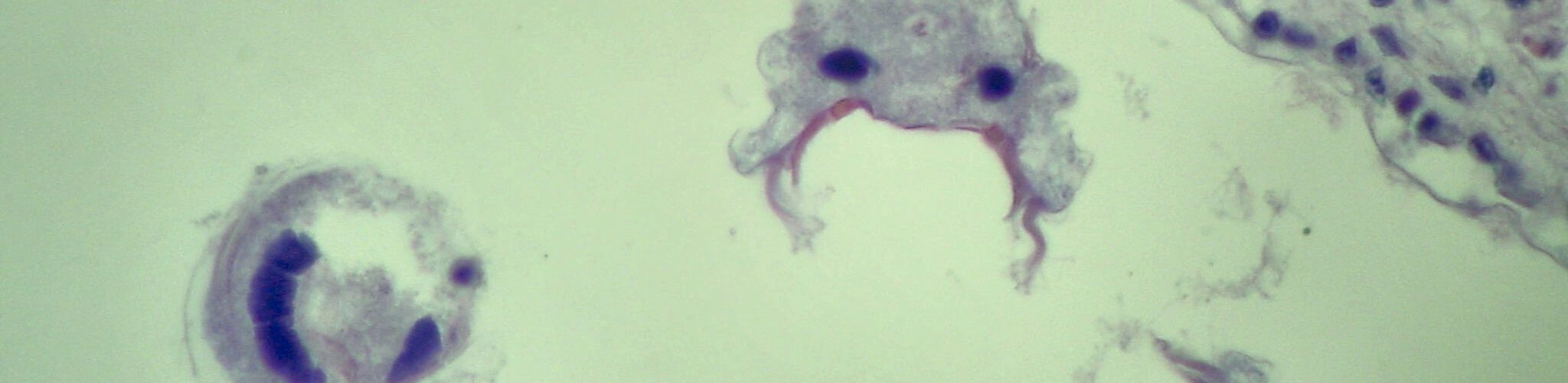
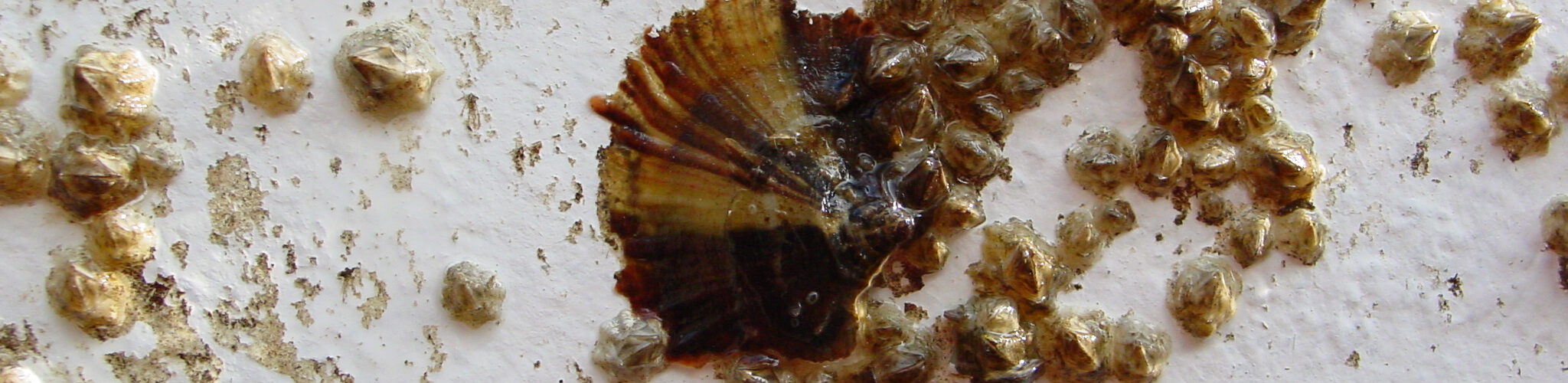
LimnoMar steht seit 30 Jahren für fundierte Forschung und Entwicklung in der aquatischen Lebenswelt, im Süßwasser wie im Meer. Als privates Forschungsinstitut hat sich LimnoMar umfangreiche Erfahrungen im Bereich des Bewuchs-Schutzes auf Schiffen und Booten erworben. Hierzu gehörten vor allem:
- Die Erforschung neuer Technologien
- Einschätzung und Bewertung existierender Verfahren
Die Auswirkungen der Schifffahrt und des Wassersports auf die aquatische Lebenswelt und den Menschen gewinnen immer mehr an Bedeutung. So bedarf es in Zukunft eines vorausschauenden Biofouling Managements. Daher sind für neue Technologien existierende und zukünftige gesetzlichen Bestimmungen im Sinne eines umfassenden Umweltschutzes, der Biosicherheit und der Gewährleistung eines nachhaltigen Schiffs- und Bootsbetriebs von entscheidender Bedeutung. LimnoMar berät unter diesem interdisziplinären und holistischen Ansatz, und versucht die notwendige Kommunikation zwischen Forschungseinrichtungen, Industrie, Schifffahrt und Behörden anzuregen.